 (Pimpinella anisum) An HERB belonging to the PARSLEY family that originated in India and was cultivated in ancient China and Egypt. The fruit, aniseed, is dried and used as a seasoning. The distinctive, licorice-like flavor of anise extract adds to the taste of shortbread cakes, such as pizella (Italy) and pains á l’anis (France). Anise is used in certain candies. Crushed aniseed together with cinnamon and coriander is used to make a liqueur, anisette. Chopped anise leaves have been used in pickled vegetables and soups.
(Pimpinella anisum) An HERB belonging to the PARSLEY family that originated in India and was cultivated in ancient China and Egypt. The fruit, aniseed, is dried and used as a seasoning. The distinctive, licorice-like flavor of anise extract adds to the taste of shortbread cakes, such as pizella (Italy) and pains á l’anis (France). Anise is used in certain candies. Crushed aniseed together with cinnamon and coriander is used to make a liqueur, anisette. Chopped anise leaves have been used in pickled vegetables and soups.
Tuesday, May 31, 2011
Anise and Human Health
 (Pimpinella anisum) An HERB belonging to the PARSLEY family that originated in India and was cultivated in ancient China and Egypt. The fruit, aniseed, is dried and used as a seasoning. The distinctive, licorice-like flavor of anise extract adds to the taste of shortbread cakes, such as pizella (Italy) and pains á l’anis (France). Anise is used in certain candies. Crushed aniseed together with cinnamon and coriander is used to make a liqueur, anisette. Chopped anise leaves have been used in pickled vegetables and soups.
(Pimpinella anisum) An HERB belonging to the PARSLEY family that originated in India and was cultivated in ancient China and Egypt. The fruit, aniseed, is dried and used as a seasoning. The distinctive, licorice-like flavor of anise extract adds to the taste of shortbread cakes, such as pizella (Italy) and pains á l’anis (France). Anise is used in certain candies. Crushed aniseed together with cinnamon and coriander is used to make a liqueur, anisette. Chopped anise leaves have been used in pickled vegetables and soups.
What is anion?
 A negatively charged ion. Anions are the opposite of CATIONS, which carry positive charges. Important anions are formed when weak acids ionize. Anions, together with their cation counterparts, occur in blood and are called electrolytes. They are required to maintain the appropriate effective concentration of ions and PROTEINS in the
A negatively charged ion. Anions are the opposite of CATIONS, which carry positive charges. Important anions are formed when weak acids ionize. Anions, together with their cation counterparts, occur in blood and are called electrolytes. They are required to maintain the appropriate effective concentration of ions and PROTEINS in theblood. Key anions in blood are chloride (Cl–), phosphate (H2PO4–), and bicarbonate (HCO3
–).
Chloride (Cl–) is the predominant anion in body fluids. Neither chloride nor phosphate can be made by the body; they are essential nutrients to be supplied by the diet. Phosphate and bicarbonate ions help buffer blood at nearly a constant pH. These anions are examples of “conjugate bases,” formed when weak acids ionize.
What is angiotensin?
 A protein-like hormone formed in the blood that raises blood pressure. Angiotensin contracts the muscles of CAPILLARIES and ARTERIES (vasopressor), which increases resistance for blood flow. Angiotensin is liberated by the action of RENIN, an ENZYME formed by the kidneys, on a serum PROTEIN (angiotensinogen) produced by the liver. The release of renin by the kidneys is triggered when they experience lowered blood flow, for example, due to dehydration. Angiotensin also plays an important role in the regulation of blood pressure by stimulating the ADRENAL GLANDS to secrete ALDOSTERONE. Aldosterone, in turn, promotes SODIUM retention and water retention by the kidneys, to help regulate water balance.
A protein-like hormone formed in the blood that raises blood pressure. Angiotensin contracts the muscles of CAPILLARIES and ARTERIES (vasopressor), which increases resistance for blood flow. Angiotensin is liberated by the action of RENIN, an ENZYME formed by the kidneys, on a serum PROTEIN (angiotensinogen) produced by the liver. The release of renin by the kidneys is triggered when they experience lowered blood flow, for example, due to dehydration. Angiotensin also plays an important role in the regulation of blood pressure by stimulating the ADRENAL GLANDS to secrete ALDOSTERONE. Aldosterone, in turn, promotes SODIUM retention and water retention by the kidneys, to help regulate water balance.
Saturday, April 30, 2011
What is pernicious anemia?
 A form of ANEMIA caused either by a dietary deficiency of VITAMIN B12 or by inadequate B12 absorption. It is characterized by quite large red blood cells (macrocytic) that are overloaded in the hemoglobin (hyperchromic). Low vitamin B12 consumption is a concern for strict VEGETARIANS who avoid meat and meat products. Pernicious anemia is also caused by inadequate vitamin B12 uptake. Normally, the gastric lining secretes a PROTEIN called INTRINSIC FACTOR that’s needed to specifically bind vitamin B12. Because this protein is required for vitamin B12 absorption by the intestine, inadequate intrinsic factor production, even with adequate dietary B12, can cause pernicious anemia.
A form of ANEMIA caused either by a dietary deficiency of VITAMIN B12 or by inadequate B12 absorption. It is characterized by quite large red blood cells (macrocytic) that are overloaded in the hemoglobin (hyperchromic). Low vitamin B12 consumption is a concern for strict VEGETARIANS who avoid meat and meat products. Pernicious anemia is also caused by inadequate vitamin B12 uptake. Normally, the gastric lining secretes a PROTEIN called INTRINSIC FACTOR that’s needed to specifically bind vitamin B12. Because this protein is required for vitamin B12 absorption by the intestine, inadequate intrinsic factor production, even with adequate dietary B12, can cause pernicious anemia.Pernicious anemia affects the nervous system as well as the blood. Symptoms include memory loss, weakness, personality and mood swings, and numbness and tingling in the hands and feet. If this anemia continues unchecked, nerve damage may be irreversible. Pernicious anemia is most common in males between the ages of 40 and 65 years who have a family history of the condition. Treatment for intrinsic factor defect involves vitamin B12 injections. Oral doses of vitamin B12 can remedy dietary deficiencies when intrinsic factor production is normal.
What is aplastic anemia ?

A form of ANEMIA in which the numbers of RED BLOOD CELLS as well as white cells are reduced. This type of anemia is caused by exposure to chemicals (such as solvents), toxic heavy metals, some drugs like chloramphenicol, or ionizing radiation (like X rays). Radiation therapy, chemotherapy, and lead poisoning can damage bone marrow, thus reducing red blood cell production. Both the blood platelet count and immunity decline, with a concomitant increased susceptibility to infection. Destruction of the bone marrow is potentially life-threatening.
Why Do You Have Anemia?
 A condition characterized by subnormal levels of HEMOGLOBIN, the oxygen-binding PROTEIN in blood. Half a million Americans are at risk for anemia, including 40 percent of pregnant women, pre-menopausal women, vegans (those who eat no animal products), adolescents relying on JUNK FOOD diets, infants, and children with inadequate diets.
A condition characterized by subnormal levels of HEMOGLOBIN, the oxygen-binding PROTEIN in blood. Half a million Americans are at risk for anemia, including 40 percent of pregnant women, pre-menopausal women, vegans (those who eat no animal products), adolescents relying on JUNK FOOD diets, infants, and children with inadequate diets.Anemia may result from either an inadequate number of RED BLOOD CELLS (erythrocytes) or an abnormally low hemoglobin content of red blood cells. With deficient functional red blood cells, the oxygen supply to tissues is inadequate for optimal RESPIRATION, causing shortness of breath, FATIGUE, weakness, pallor, headache, and lowered resistance to infection. There are two general types of anemia based on red blood cell size. Megaloblastic anemia is characterized by large red blood cells; their shortened life span results in a decreased number of cells. Microcytic anemia is characterized by small red blood cells with reduced hemoglobin content. Many nutritional deficiencies lead to anemia. Inadequate dietary IRON, COPPER, FOLIC ACID, PROTEIN,
VITAMIN B6, vitamin B12, VITAMIN C, VITAMIN A, VITAMIN E, and RIBOFLAVIN can cause this condition. Each of these nutrients is required for the production of red blood cells (ERYTHROPOIESIS). Iron deficiency anemia is the most common diet-related anemia in the United States and it represents the last stage of iron deficiency. It is characterized by small, pale red blood cells (microcytic anemia), due to chronic blood loss or inadequate iron intake. Symptoms include FATIGUE, pallor, and shortness of breath. Studies of the nutritional status of developed nations have routinely found up to 30 percent of a population with iron deficiency. Groups that are at highest risk are children under the age of two years, teenage women, pregnant women, and the elderly. Pregnancy drastically increases the requirement of iron. In terms of blood loss the most common causes of iron deficiency are excessive bleeding during menstruation and intestinal bleeding due to parasites, ulcers, or malignancy. Iron deficiency can be caused by impaired iron uptake by the intestine, due to a lack of stomach acid (ACHLORHYDRIA) or from chronic DIARRHEA. With iron deficiency, the resulting anemia can be treated by iron supplementation.
Deficiencies of either folacin or vitamin B12 can cause anemia because each is essential for DNA synthesis and deficiencies impair erythrocyte production. Folic acid deficiency is much more common because folic acid stores in the body are small, yet folic acid participates in many biosynthetic reactions. On the other hand, vitamin B12 is stored in the LIVER, and only trace amounts are required daily for a few specific functions. Anemia due to inadequate folic acid and vitamin B12 produces large (macrocytic) cells with a short life span. This form of anemia can occur when intake of fresh vegetables is very limited, or when the need for folic acid outstrips intake, as may occur during pregnancy or in ALCOHOLISM. Treatment with folic acid can ameliorate megaloblastic anemia, yet mask an underlying vitamin B12 deficiency. This point emphasizes that treatment of anemia requires expert medical supervision.
Anemia can also indicate a serious condition unrelated to diet. Non-nutritional causes of anemia include chronic blood loss and congenital defects in red blood cell formation, such as thalassemia or sickle cell anemia, due to mutant hemoglobins, and spherocytosis (spherical red blood cells). Hemolytic anemia is the result of excessive hemolysis (destruction of red blood cells) in susceptible people exposed to bacterial toxins, toxic chemicals, or drugs that may produce JAUNDICE.
Anemia also may result from reduced nutrient uptake due to the presence of parasites and chronic infections, gastrointestinal disease or bowel resection
Thursday, March 31, 2011
Foods and anaphylaxis
 An extreme reaction of the immune system in response to exposure to foreign substances. Insect bites, drugs, injected serum, and certain foods can create anaphylaxis. This abnormal response or immediate hypersensitivity is usually very rapid in susceptible individuals who may have been sensitized by previous exposure, and may produce shock (“anaphylactic shock”). The massive release of histamines and other inflammatory agents leads to spasming of smooth muscles, especially those of the air passageways, and to widespread swelling due to the increased water leaking out of capillaries. Symptoms range from asthma to fever, itching, hives, and flushed skin in mild cases, to chest constriction, irregular pulse, painful, labored breathing, and convulsions in severe cases. Anaphylaxis can be life-threatening and may require emergency room care.
An extreme reaction of the immune system in response to exposure to foreign substances. Insect bites, drugs, injected serum, and certain foods can create anaphylaxis. This abnormal response or immediate hypersensitivity is usually very rapid in susceptible individuals who may have been sensitized by previous exposure, and may produce shock (“anaphylactic shock”). The massive release of histamines and other inflammatory agents leads to spasming of smooth muscles, especially those of the air passageways, and to widespread swelling due to the increased water leaking out of capillaries. Symptoms range from asthma to fever, itching, hives, and flushed skin in mild cases, to chest constriction, irregular pulse, painful, labored breathing, and convulsions in severe cases. Anaphylaxis can be life-threatening and may require emergency room care.
What is anaerobic
 Cellular processes that do not require oxygen. Energy can be produced in cells without oxygen. Anaerobic GLYCOLYSIS refers to an energy yielding process by which ATP, the energy currency of the cell, is produced from GLUCOSE without the participation of oxygen. As an example, skeletal muscle produces LACTIC ACID and ATP from glucose when oxygen supplied to muscle is inadequate to meet energy needs during strenuous physical exertion.
Cellular processes that do not require oxygen. Energy can be produced in cells without oxygen. Anaerobic GLYCOLYSIS refers to an energy yielding process by which ATP, the energy currency of the cell, is produced from GLUCOSE without the participation of oxygen. As an example, skeletal muscle produces LACTIC ACID and ATP from glucose when oxygen supplied to muscle is inadequate to meet energy needs during strenuous physical exertion.Accumulated lactic acid is then converted back to glucose during the recovery period following EXERCISE when the oxygen supply is again adequate.
Anaerobic processes are important for certain bacteria as well. Anaerobic bacteria in the intestine grow without oxygen and block the growth of potential disease-producing microorganisms. Anaerobic fermentation of SUGAR by yeast yields alcohol-containing products such as WINE and BEER.
What is anabolism (biosynthesis)?
 Processes involved in synthesizing the molecules needed for cellular growth and maintenance. Thus the formation of PROTEIN, DNA, RNA, LIPID, CARBOHYDRATE, FAT, and GLYCOGEN are anabolic processes. Anabolism consumes chemical energy in the form of ATP and NADPH (a reducing agent), which are supplied by CATABOLISM, the energy-yielding oxidative processes involved in degradation. Optimal function and health rely upon a balance of anabolic and catabolic processes (homeostasis). These two branches of metabolism are controlled by the ENDOCRINE SYSTEM, which in turn responds to external influences such as diet. Anabolic processes require small building blocks supplied by breaking down STARCH, PROTEIN, and FAT in foods to build larger molecules. GLYCEROL and FATTY ACIDS are the subunits of fat; AMINO ACIDS yield proteins; and glucose yields glycogen. Fat and carbohydrate degradation provides an energized form of ACETIC ACID (acetyl CoA) to synthesize fatty acids and cholesterol. Other specialized products are also assembled from several different types of smaller precursors. For example, heme, the iron-containing pigment of the oxygen transport protein HEMOGLOBIN, is synthesized from an amino acid (GLYCINE) and SUCCINIC ACID, a common intermediate in energy-producing pathways.
Processes involved in synthesizing the molecules needed for cellular growth and maintenance. Thus the formation of PROTEIN, DNA, RNA, LIPID, CARBOHYDRATE, FAT, and GLYCOGEN are anabolic processes. Anabolism consumes chemical energy in the form of ATP and NADPH (a reducing agent), which are supplied by CATABOLISM, the energy-yielding oxidative processes involved in degradation. Optimal function and health rely upon a balance of anabolic and catabolic processes (homeostasis). These two branches of metabolism are controlled by the ENDOCRINE SYSTEM, which in turn responds to external influences such as diet. Anabolic processes require small building blocks supplied by breaking down STARCH, PROTEIN, and FAT in foods to build larger molecules. GLYCEROL and FATTY ACIDS are the subunits of fat; AMINO ACIDS yield proteins; and glucose yields glycogen. Fat and carbohydrate degradation provides an energized form of ACETIC ACID (acetyl CoA) to synthesize fatty acids and cholesterol. Other specialized products are also assembled from several different types of smaller precursors. For example, heme, the iron-containing pigment of the oxygen transport protein HEMOGLOBIN, is synthesized from an amino acid (GLYCINE) and SUCCINIC ACID, a common intermediate in energy-producing pathways.Growth and an anabolic state, seen as an increase in body mass and muscle mass, occur during childhood, adolescence, pregnancy, and strenuous physical activity, such as body building. The weight gained in these situations represents increased protein, bone, or fat, not fluids. Increased fat stores and accumulated body fat represent stored surplus energy in adults and can result from too little exercise, the over-consumption of FOOD, heredity, or a combination of the above factors.
Monday, February 28, 2011
Anabolic Steroids

A family of steroids related to the male sex hormone TESTOSTERONE. These are classified as prescription drugs used to make up for hormone imbalance and deficiencies. However, synthetic analogs of testosterone have been obtained illegally by athletes and by teenage males to build muscles, and the U.S. FDA has described steroid abuse as a drug epidemic. While testosterone stimulates growth during adolescence, the synthetic derivatives can cause many side effects. Athletes compound this unsafe practice by “stacking” anabolic steroids—taking a combination of brands at 10 to 100 times the recommended doses for weeks at a time.
In men, the side effects of anabolic steroid use include lowered sperm count, enlarged prostate gland, shrinking testicles, balding, and enlarged breasts. If taken before puberty, anabolic steroids can stunt growth. These effects seem to be reversible if anabolic steroids have been used for a short time. Some women body builders also use steroids to build muscle. Side effects in women do not seem to be reversible: masculinization, including increased muscles, increased size of clitoris, growth of facial hair, a deepening voice, shrinkage of breast size, uterine atrophy, and menstrual irregularities. Severe cases of acne and bouts of rage are signs of anabolic steroid use, especially in males. Anabolic steroid use can have more subtle, longterm detrimental effects; damage may show up years later as a HEART ATTACK, high blood pressure, CANCER, and LIVER damage in both men and women.
Amylose
 A water-soluble form of STARCH found in seeds, tubers, and root vegetables. It is made up of long chains of GLUCOSE units, and often contains a thousand or more glucose units. Amylose differs from the other prevalent form of starch, AMYLOPECTIN, which is highly branched. Amylose forms large spiral configurations when dissolved in water and can react with iodide to form a characteristic blue-purple pigment. Amylopectin and amylose occur together in starch, and the relative amounts vary depending on the plant sources. During digestion, AMYLASE breaks down amylose to maltose, a disaccharide composed of two glucose units. An intestinal enzyme, MALTASE, then hydrolyzes maltose to the simple sugar glucose, the ultimate product of starch digestion.
A water-soluble form of STARCH found in seeds, tubers, and root vegetables. It is made up of long chains of GLUCOSE units, and often contains a thousand or more glucose units. Amylose differs from the other prevalent form of starch, AMYLOPECTIN, which is highly branched. Amylose forms large spiral configurations when dissolved in water and can react with iodide to form a characteristic blue-purple pigment. Amylopectin and amylose occur together in starch, and the relative amounts vary depending on the plant sources. During digestion, AMYLASE breaks down amylose to maltose, a disaccharide composed of two glucose units. An intestinal enzyme, MALTASE, then hydrolyzes maltose to the simple sugar glucose, the ultimate product of starch digestion.
Amylopectin
 The water-insoluble form of STARCH. Plants synthesize this very long chain of GLUCOSE units as a storage form of energy, often to nurture the future embryo, seedling or sprout. It is often the major form of starch and it possesses a highly branched, bushy structure resembling liver GLYCOGEN (animal starch). In contrast, AMYLOSE is made up of single straight chains of glucose units.
The water-insoluble form of STARCH. Plants synthesize this very long chain of GLUCOSE units as a storage form of energy, often to nurture the future embryo, seedling or sprout. It is often the major form of starch and it possesses a highly branched, bushy structure resembling liver GLYCOGEN (animal starch). In contrast, AMYLOSE is made up of single straight chains of glucose units.Amylopectin forms a paste in hot water. Starch occurs in seeds, tubers, and root vegetables as both amylopectin and amylose, although the ratio of two forms varies with the source. Cooking softens starch granules, making them available to DIGESTION by AMYLASE. The ultimate product of amylopectin digestion is GLUCOSE. Commercial processing converts starch to glucose, then to HIGHFRUCTOSE CORN SYRUP, a major sweetener
Monday, January 31, 2011
Ammonia (NH3) and Human Health

The nitrogen waste produced primarily from AMINO ACID metabolism. Ammonia is highly toxic to the nervous system and the brain. It may interfere with metabolic processes required for energy production in the brain. Normally the brain transforms ammonia into GLUTAMINE, a safe, neutral amino acid released into the bloodstream. Next, glutamine is absorbed by the intestine, which releases the ammonia for disposal by the LIVER. Normally the liver very efficiently metabolizes ammonia to UREA, the ultimate nontoxic waste product, via the UREA CYCLE to keep the level of ammonia in the blood at very low levels. Urea is excreted safely in urine. Ammonia is also produced in the intestinal tract by bacteria. Ammonia is absorbed by the intestine and transported directly via the portal vein to the liver for disposal. Liver disease, such as CIRRHOSIS, reduces urea production and leads to elevated blood levels of ammonia (ammonemia), which causes neurological abnormalities. Genetic defects in the ammoniadisposal mechanism of the urea cycle generally lead to brain damage.
What is amino sugars
 A family of nitrogen-containing sugars. Cells attach NITROGEN to the simple sugars GLUCOSE and GALACTOSE to produce GLUCOSAMINE and galactosamine, respectively. These and similar amino sugars are used to produce carbohydratecontaining proteins (GLYCOPROTEINS) that coat cell surfaces, and for structural materials (MUCOPOLYSACCHARIDES) that help form the matrix of cartilage for ligaments and joints.
A family of nitrogen-containing sugars. Cells attach NITROGEN to the simple sugars GLUCOSE and GALACTOSE to produce GLUCOSAMINE and galactosamine, respectively. These and similar amino sugars are used to produce carbohydratecontaining proteins (GLYCOPROTEINS) that coat cell surfaces, and for structural materials (MUCOPOLYSACCHARIDES) that help form the matrix of cartilage for ligaments and joints.
Understanding Amino Acids
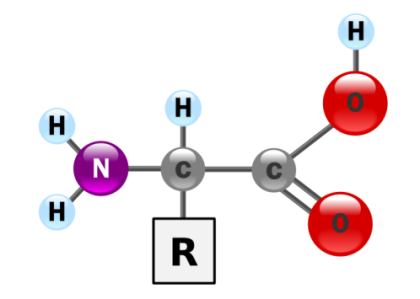 Small organic acids that serve as raw materials of PROTEINS. The 50,000 to 100,000 different proteins in the body are combinations of just 20 different types of amino acids. During protein synthesis, amino acids are linked together like beads on a string to form long chains (polypeptides). Each type of protein possesses a unique amino acid sequence, specified by the cell’s genes.
Small organic acids that serve as raw materials of PROTEINS. The 50,000 to 100,000 different proteins in the body are combinations of just 20 different types of amino acids. During protein synthesis, amino acids are linked together like beads on a string to form long chains (polypeptides). Each type of protein possesses a unique amino acid sequence, specified by the cell’s genes.As the name implies, each amino acid possesses an amino group and a carboxylic acid functional group, and therefore amino acids behave as both ACIDS and BASES. They also possess side chains with different properties. For example, certain amino acids, like ASPARTIC ACID and GLUTAMIC ACID, are acidic; others like ARGININE and LYSINE are basic;
METHIONINE and CYSTEINE contain SULFUR. Another group repels water and has the branched chains:
VALINE, LEUCINE, and ISOLEUCINE. Just as hands and feet are mirror images of each other, amino acids occur as mirror-image forms (optical isomers). The left-hand forms are desigamino nated as “L,” and the right-handed opposites are designated as “D.” Only L-amino acids are supplied by food and synthesized in the body, and only the “L” forms occur in proteins. Therefore, unless indicated otherwise, an amino acid can be assumed to be the “L-” form when mentioned in nutrition literature. The only common amino acid that does not exist as optical isomers is glycine, the simplest of amino acids.
DIGESTION of food proteins releases amino acids, which are absorbed in the INTESTINE. Depending on the person’s body size and the type of protein that is consumed, 55 g to 65 g of protein a day supplies adequate amino acids for an adult. Few Americans are likely to be protein-deficient, because the typical U.S. diet generally supplies twice as much protein as needed. With a varied diet, neither a meat eater nor a knowledgeable VEGETARIAN needs extra protein to obtain adequate amino acids.
MEAT, FISH, POULTRY, and DAIRY products like EGGS are the best sources of essential amino acids. Proteins that provide ample amounts of essential amino acids are said to be COMPLETE PROTEINS. Several plant proteins approach the quality of animal protein: soy, AMARANTH and QUINOA are examples. However, most plant proteins are deficient in at least one essential amino acid. For example, LEGUMES are low in methionine; CORN is low in lysine. These foods can be balanced during the day by eating “complementary” protein foods that provide ample amounts of those amino acids deficient in another food. Surplus dietary amino acids may be used for energy, and amino acids from the breakdown of cellular protein can be important fuel sources when food intake is inadequate. After 12 to 24 hours without food, MUSCLE protein breaks down rapidly, releasing amino acids into the bloodstream and processing them in the LIVER. The liver removes NITROGEN and converts it to UREA, while converting the amino acids to GLUCOSE and releasing it into the bloodstream to maintain blood sugar levels. In this way, most amino acids can contribute to blood glucose; consequently, muscle protein can help fuel the brain during STARVATION when the glucose supply becomes critical.
Ten amino acids are designated as dietary “nonessential” amino acids because they are synthesized by the body and do not need to be supplied in food. On the other hand, the diet must provide the other eight amino acids to prevent malnutrition. These dietary “essential” amino acids are lysine, valine, PHENYLALANINE, TRYPTOPHAN, isoleucine, leucine, METHIONINE, and THREONINE. Two other amino acids may be conditionally essential. HISTIDINE may not be formed in adequate amounts by infants and growing children, and arginine may be inadequately synthesized by adults with liver disease and by BREAST-FEEDING mothers.
Amino acids like phenylalanine and arginine, thought to stimulate GROWTH HORMONE release and thus promote FAT loss, are neither safe nor effective methods for weight control. Large amounts (several grams per day) of single amino acids used as supplements or additives, can drastically affect the body and damage the KIDNEYS. The therapeutic use of amino acids is still in experimental stages. The U.S. FDA removed amino acids from the GENERALLY RECOGNIZED AS SAFE list of FOOD ADDITIVES, and it is prudent to consult a health care provider before supplementing with individual amino acids.
Subscribe to:
Posts (Atom)
