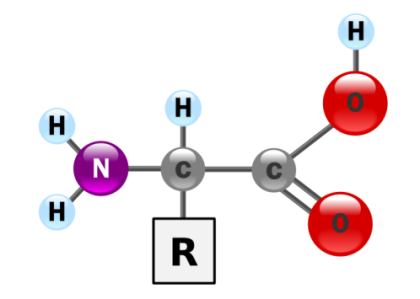
Small organic acids that serve as raw materials of PROTEINS. The 50,000 to 100,000 different proteins in the body are combinations of just 20 different types of amino acids. During protein synthesis, amino acids are linked together like beads on a string to form long chains (polypeptides). Each type of protein possesses a unique amino acid sequence, specified by the cell’s genes.
As the name implies, each amino acid possesses an amino group and a carboxylic acid functional group, and therefore amino acids behave as both ACIDS and BASES. They also possess side chains with different properties. For example, certain amino acids, like ASPARTIC ACID and GLUTAMIC ACID, are acidic; others like ARGININE and LYSINE are basic;
METHIONINE and CYSTEINE contain SULFUR. Another group repels water and has the branched chains:
VALINE, LEUCINE, and ISOLEUCINE. Just as hands and feet are mirror images of each other, amino acids occur as mirror-image forms (optical isomers). The left-hand forms are desigamino nated as “L,” and the right-handed opposites are designated as “D.” Only L-amino acids are supplied by food and synthesized in the body, and only the “L” forms occur in proteins. Therefore, unless indicated otherwise, an amino acid can be assumed to be the “L-” form when mentioned in nutrition literature. The only common amino acid that does not exist as optical isomers is glycine, the simplest of amino acids.
DIGESTION of food proteins releases amino acids, which are absorbed in the INTESTINE. Depending on the person’s body size and the type of protein that is consumed, 55 g to 65 g of protein a day supplies adequate amino acids for an adult. Few Americans are likely to be protein-deficient, because the typical U.S. diet generally supplies twice as much protein as needed. With a varied diet, neither a meat eater nor a knowledgeable VEGETARIAN needs extra protein to obtain adequate amino acids.
MEAT, FISH, POULTRY, and DAIRY products like EGGS are the best sources of essential amino acids. Proteins that provide ample amounts of essential amino acids are said to be COMPLETE PROTEINS. Several plant proteins approach the quality of animal protein: soy, AMARANTH and QUINOA are examples. However, most plant proteins are deficient in at least one essential amino acid. For example, LEGUMES are low in methionine; CORN is low in lysine. These foods can be balanced during the day by eating “complementary” protein foods that provide ample amounts of those amino acids deficient in another food. Surplus dietary amino acids may be used for energy, and amino acids from the breakdown of cellular protein can be important fuel sources when food intake is inadequate. After 12 to 24 hours without food, MUSCLE protein breaks down rapidly, releasing amino acids into the bloodstream and processing them in the LIVER. The liver removes NITROGEN and converts it to UREA, while converting the amino acids to GLUCOSE and releasing it into the bloodstream to maintain blood sugar levels. In this way, most amino acids can contribute to blood glucose; consequently, muscle protein can help fuel the brain during STARVATION when the glucose supply becomes critical.
Ten amino acids are designated as dietary “nonessential” amino acids because they are synthesized by the body and do not need to be supplied in food. On the other hand, the diet must provide the other eight amino acids to prevent malnutrition. These dietary “essential” amino acids are lysine, valine, PHENYLALANINE, TRYPTOPHAN, isoleucine, leucine, METHIONINE, and THREONINE. Two other amino acids may be conditionally essential. HISTIDINE may not be formed in adequate amounts by infants and growing children, and arginine may be inadequately synthesized by adults with liver disease and by BREAST-FEEDING mothers.
Amino acids like phenylalanine and arginine, thought to stimulate GROWTH HORMONE release and thus promote FAT loss, are neither safe nor effective methods for weight control. Large amounts (several grams per day) of single amino acids used as supplements or additives, can drastically affect the body and damage the KIDNEYS. The therapeutic use of amino acids is still in experimental stages. The U.S. FDA removed amino acids from the GENERALLY RECOGNIZED AS SAFE list of FOOD ADDITIVES, and it is prudent to consult a health care provider before supplementing with individual amino acids.


 A family of nitrogen-containing sugars. Cells attach NITROGEN to the simple sugars GLUCOSE and GALACTOSE to produce GLUCOSAMINE and galactosamine, respectively. These and similar amino sugars are used to produce carbohydratecontaining proteins (GLYCOPROTEINS) that coat cell surfaces, and for structural materials (MUCOPOLYSACCHARIDES) that help form the matrix of cartilage for ligaments and joints.
A family of nitrogen-containing sugars. Cells attach NITROGEN to the simple sugars GLUCOSE and GALACTOSE to produce GLUCOSAMINE and galactosamine, respectively. These and similar amino sugars are used to produce carbohydratecontaining proteins (GLYCOPROTEINS) that coat cell surfaces, and for structural materials (MUCOPOLYSACCHARIDES) that help form the matrix of cartilage for ligaments and joints.
 Small organic acids that serve as raw materials of PROTEINS. The 50,000 to 100,000 different proteins in the body are combinations of just 20 different types of amino acids. During protein synthesis, amino acids are linked together like beads on a string to form long chains (polypeptides). Each type of protein possesses a unique amino acid sequence, specified by the cell’s genes.
Small organic acids that serve as raw materials of PROTEINS. The 50,000 to 100,000 different proteins in the body are combinations of just 20 different types of amino acids. During protein synthesis, amino acids are linked together like beads on a string to form long chains (polypeptides). Each type of protein possesses a unique amino acid sequence, specified by the cell’s genes.